Current estimates from the Centre for Disease Control and Prevention (CDC) state that around 1.2 million Americans are HIV positive, with about 20% of those (240,000) unaware that they are HIV positive. Every year in the US, there are approximately 50,000 new incidences of HIV transmission.
Whilst all formal diagnoses of HIV must be verified by a doctor, the OraQuick Test is a simple piece of equipment that will hopefully reduce the transmission rate of HIV by shortening the time of infection to time of diagnosis period. Then sufferers can obtain advice, anti-retroviral treatment and the care that they require as soon as possible. It is, as the New York Times stated, "… another step in the normalization of a disease that was once seen as a mark of shame and a death sentence".
However, the effectiveness of such a test, however good its intentions, is of extreme importance. Most medical tests carry some slight margin of error and the OraQuick Test is not exclusive to this fact.
How Accurate is the OraQuick Test?
Researchers found that that 92% of people who are HIV positive received a positive test result. This is called the "sensitivity" of the test. They also found that 99.98% of people who were HIV negative received a negative test result. This is called the "specificity" of the test.
In terms of numbers, this means that about 2 in every 25 people who have HIV will receive a false negative test, and 2 in every 10,000 people without HIV will receive a false positive result.
Whilst the sensitivity and specificity for the OraQuick Test may seem reasonably high, there is an additional result that is of importance here that we must consider (anyone who has done an undergraduate course in probability knows what's coming next).
Given that a person receives a positive test result, what is the probability that the person is actually HIV positive?
Statistics and HIV Screening
To answer this question, we can use the wonderful tool of Bayes' Theorem (I previously wrote an article on this for Significance in September 2011). In the context of HIV testing, the probability that someone is truly HIV positive (denoted "HIV+"), given they have a positive test result (denoted "Pos. Test"), is written as
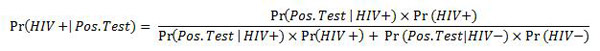
Now this might look confusing for some, but it's a very simple calculation. We already know that Pr(Pos. Test | HIV+) = 0.92, we know that Pr(Neg. Test | HIV -) = 0.9998 and we know that in America's population of over 300 million people (at the time of writing, 313,889,087), there about 1.2 million Americans who are HIV positive. So Pr(HIV+) is 0.00382 (also written as 0.382%).
By plugging these numbers into our formula above, we get the following answer:
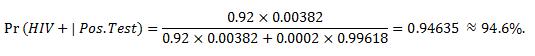
So given that someone receives a positive test result from their OraQuick Test, there is in fact a 94.6% chance that they truly are HIV positive, or conversely, a 5.4% chance they truly are HIV negative.
In HIV drug testing, this is one of the most important quantities to be aware of. Incorrect diagnosis of HIV can carry severe consequences; one of the reasons the FDA took so long to approve the OraQuick Test is that they were worried persons who found out they were HIV positive outside of a designated facility to offer direct care and attention were more prone to suicide.
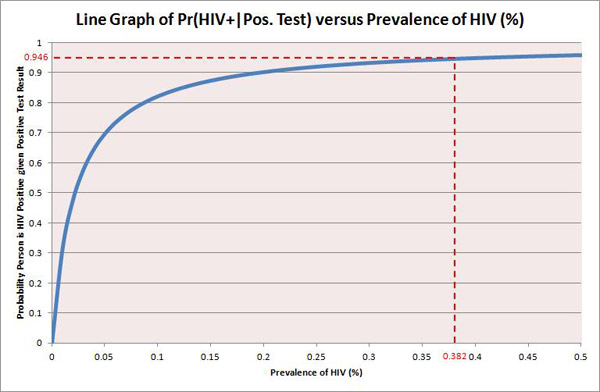
I have come across many examples of medical tests where, despite their apparently high figures of sensitivity and specificity, the probability of a person having the disease given a positive test result is extremely low. This is often dependent on the rarity of the disease being tested for. For example, Figure 1 above shows a graph of Pr(HIV+| Pos. Test) versus the prevalence of HIV in a population. As the prevalence of HIV decreases (i.e. the disease becomes rarer), the probability of someone being truly HIV positive given their positive test result decreases dramatically. So for other diseases much rarer than HIV, or indeed in populations where HIV is rarer than the US, more careful and rigorous testing is required.
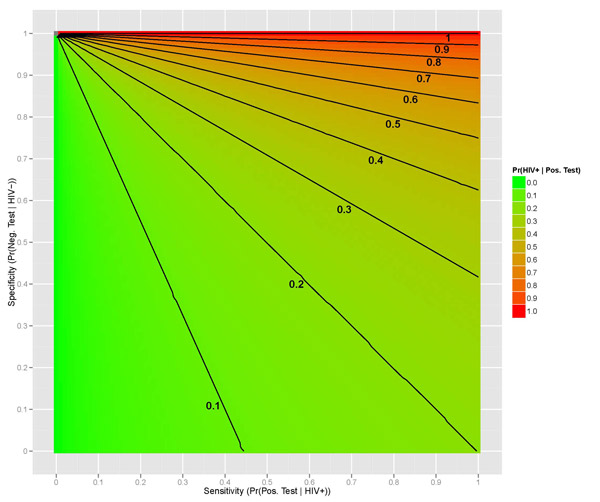
We can even show how this probability of interest changes with the level of sensitivity and specificity. Assuming that the prevalence of HIV in the US is unchanged (0.00382, or 0.382%), we can observe a surface that displays the probability of a person being truly HIV positive given their positive test result (Figure 2 below), given values of a test's sensitivity and specificity. As you can see, we require extremely high levels of both sensitivity and specificity in order to have an accurate and reliable HIV test (at least 90% sensitivity and over 99% specificity); anything less than that warrants the test virtually worthless. The blue dot indicates the 0.946 calculated previously with 92% sensitivity and 99.98% specificity.
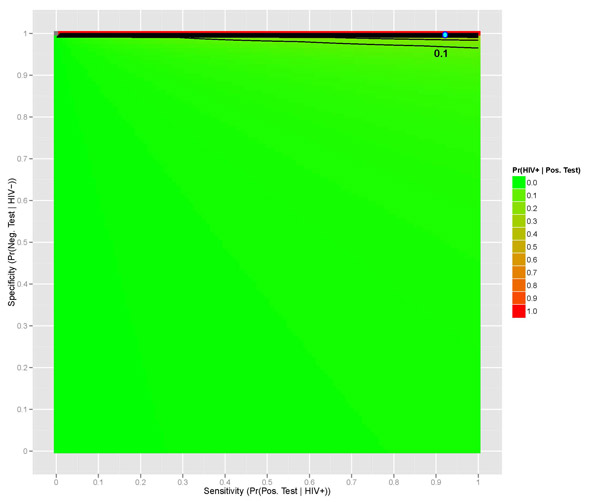
Even if we increase the prevalence of HIV to be something as dangerously high as 0.2, or 20%, we still require high levels of sensitivity and more so specificity in order to have a truly accurate test (Figure 3 below).
An excellent illustration of the problems associated with HIV screening is available from the Understanding Uncertainty webpage, created by Professor David Spiegelhalter, Winton Professor of the Public Understanding of Risk at the University of Cambridge, and colleagues. Their interactive representation of disease screening allows you to create your own tests with particular sensitivity, specificity and disease prevalence, and is definitely worth exploring.
Future Progress
As well as the commercial release of the OraQuick Test, OraSure have created a 24-hour toll-free hotline in order to provide advice on HIV, how to use the test, interpret results and even provide direct referral to care if required. The hotline is due to open on Monday 9th July, with a dedicated website full of resources available in October 2012, when the test itself is due to go on sale.
Reducing the stigma surrounding the disease and HIV tests, ensuring care and advice are provided to both sufferers and their families as soon as possible and – pending its success in the US – widening the access of this test to areas and countries severely affected by HIV and AIDS are all gargantuan tasks in public health. Hopefully, the availability of simple, cheap and accurate testing equipment, such as The OraQuick Test, will act as a major stepping-stone in the global fight against HIV and AIDS and make these goals all the more realistic.




