
These represent two distinct patterns of seasonal variation that have been observed in epidemiological studies. One relates to the season when the disease presents, e.g. excess winter mortality in heart, cerebrovascular and respiratory diseases, and sudden infant death syndrome, and psychiatric disorders. The other relates to the season of birth (or conception), such as excess mortality in Gambian adults born in the annual ‘hungry season’ (July-October), due mostly to infections and pregnancy related complications. Also, among people who died aged over 50 years lifespan is increased for those born in the autumn, and the risk of suicide is greater for those born in spring or early summer.
However the mechanisms underlying such seasonal patterns are not well understood and they have rarely been investigated at a molecular level.
The paper ‘Widespread seasonal gene expression reveals annual differences in human immunity and physiology’ published last week in Nature Communications by Xaquin Castro Dopico and co-authors shows for the first time that gene expression varies with the month of the year, so that genes have their own seasonal clock. It is known that many biological processes vary seasonally, for example vitamin D metabolism which has been linked to evolutionary adaption and immunological function. However, as Castro Dopico and colleagues state 'how seasons might more broadly impact the underlying molecular details of human physiology is unknown.'
Genes encode instructions which are carried out by mechanisms such as transcription into messenger RNA (mRNA) molecules and these are further translated into proteins, which execute cellular functions. Gene expression profiles can be regarded as quantitative phenotypic traits, and they constitute the most basic level at which the genotype gives rise to the phenotype. Gene expressions can be measured in terms of two-fold changes of a fluorescent ratio with respect to a baseline level, and such ratios are usually analysed in log2 scale.
 Castro Dopico et al analysed data on gene expression from over 16,000 individuals in the UK, Ireland, Germany, Iceland, USA, Australia and The Gambia – including information on more than 4,000 protein-coding mRNA expression levels in peripheral blood mononuclear cells and adipose tissue biopsies, full blood count data, and the circulating levels of inflammatory protein biomarkers.
Castro Dopico et al analysed data on gene expression from over 16,000 individuals in the UK, Ireland, Germany, Iceland, USA, Australia and The Gambia – including information on more than 4,000 protein-coding mRNA expression levels in peripheral blood mononuclear cells and adipose tissue biopsies, full blood count data, and the circulating levels of inflammatory protein biomarkers.
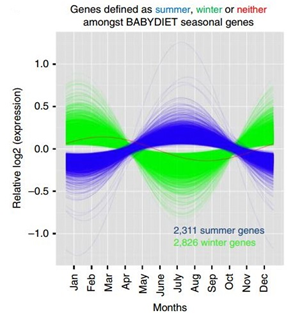
Figure 1 (right), taken from their paper, shows the fitted values of relative seasonal mRNA expression in the human immune system from 5,137 genes (out of 22,822 genes tested corresponding to ~23% of the genome), with significant winter or summer peaks. Of these, 2,826 genes had increased expression in winter, defined as December, January and February (green), 2,311 genes were upregulated in the summer, defined as June, July and August (blue), one gene (red) had a significant seasonal pattern which didn’t fall into the authors’ definition of summer and winter.
The paper establishes strong seasonal changes in the gene expression profiles of white blood cells and adipose tissue. Crucially, there is a six month shift between European and Australian patterns, reflecting the differences in season. To see if the seasonal pattern varied by latitude, the authors also compared gene expression from individuals in the UK and The Gambia. The two were very different: in the UK the peaks were mainly in April or October, whilst in The Gambia most blood cell types peaked during the rainy season (June to October), a period with the highest infectious disease burden. The same annual cycle peaking either in summer or winter was also seen in adipose tissue, showing that seasonal variation of gene expression is not restricted to blood cells.
This paper’s result 'provide a plausible mechanism to explain part of the seasonality of human disease', and point out the potential value of accounting for the season of the year for treating diseases which are known to present seasonally. For example, melatonin might be a contributory risk factor for suicide, which is more common in spring and early summer, possibly associated with increased sunshine, thus investigating the role of gene expression on melatonin production would be useful.
Castro Dopico et al conclude that the data in their paper provide 'a fundamental shift in how we conceptualize immunity in humans, and we propose that seasonal changes be more broadly considered as major determinants of human physiology.' Studies of this type might lead to prevention strategies with public health implications as their findings can be used to 'identify policy interventions aimed at reducing nature's global and local inequalities.'




